Soap has a pH level that typically ranges between 9 and 10. But what exactly does this mean? Understanding what pH is, especially in the context of soap, is crucial for selecting the right products for our skin. The pH of soap plays a significant role in its cleansing effectiveness and compatibility with our skin. Let’s dive deeper into the world of pH and soap to uncover its importance and implications for our daily skincare routine.
Understanding the pH of Soap: Everything You Need to Know
What pH is Soap?
Have you ever wondered what makes soap clean your hands so well? It’s not just the bubbles or the scent; it’s also about the pH level of the soap. In simple terms, pH is a measure of how acidic or alkaline a solution is. But what exactly is the pH of soap, and why does it matter?
The Basics of pH
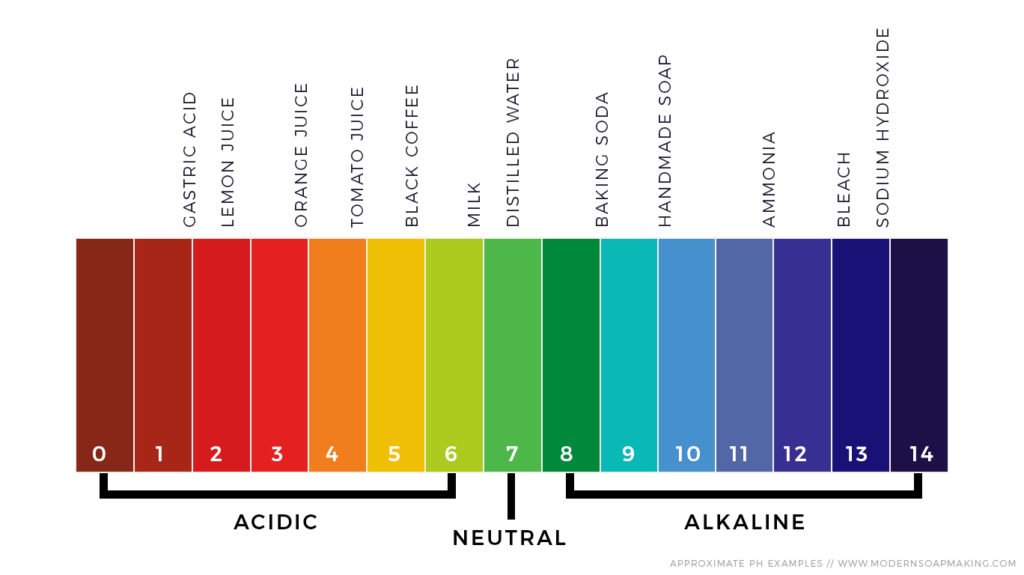
To understand the pH of soap, let’s first grasp the basics of pH. pH is a scale that ranges from 0 to 14, with 7 being neutral. Anything below 7 is considered acidic, while anything above 7 is alkaline. The lower the number, the more acidic the substance, and the higher the number, the more alkaline it is.
Acids and Bases in Soap
Soap is an interesting product because it can have both acidic and alkaline properties depending on its ingredients. Most traditional soaps are slightly alkaline with a pH ranging from 8 to 10. This alkalinity helps soap to cut through grease and oils effectively, making it a great cleaner.
Why pH Matters in Soap
The pH level of soap plays a crucial role in its effectiveness. When soap is too acidic or too alkaline, it may not work as well at cleaning. For example, if a soap is too acidic, it might not lather well or remove dirt and grime effectively. On the other hand, if it’s too alkaline, it could be harsh on the skin and strip away natural oils.
The Importance of Balance
For soap to work effectively and be gentle on your skin, it needs to have a balanced pH. The slightly alkaline nature of most soaps helps them to clean effectively without being too harsh. This balance is crucial for maintaining healthy and clean skin.
Testing the pH of Soap
So, how can you determine the pH of a soap? There are a few ways to do this, but the most common method is to use pH testing strips. These strips change color based on the acidity or alkalinity of a substance, giving you an idea of its pH level.
DIY pH Testing
If you’re feeling adventurous, you can even try testing the pH of your soap at home. Simply wet a pH strip with water, rub it on the soap, and observe the color change. Match the color to the pH scale provided with the strips to determine the approximate pH of your soap.
Implications for Different Types of Soap
Not all soaps have the same pH level, which is why it’s essential to choose the right soap for your skin type. Here’s a breakdown of different types of soaps and their typical pH ranges:
1. Handmade Soap
Handmade soaps often have a slightly higher pH due to the natural ingredients used. They typically fall in the pH range of 8-10, similar to traditional soaps.
2. Antibacterial Soap
Antibacterial soaps may have a higher pH to help kill bacteria effectively. However, using them too frequently can disrupt the skin’s natural balance.
3. Baby Soap
Baby soaps are usually more neutral in pH, around 7, to be gentle on the delicate skin of babies. It’s important to use mild soaps for infants to avoid irritation.
4. Moisturizing Soap
Moisturizing soaps often have additives like glycerin to help hydrate the skin. These soaps tend to have a pH closer to neutral to prevent drying out the skin.
The next time you reach for a bar of soap, take a moment to consider its pH. Understanding the pH of soap can help you choose the right product for your skin’s needs. Remember, a balanced pH is key to effective cleaning without being too harsh. So, whether you prefer handmade, antibacterial, baby, or moisturizing soap, make sure it’s pH-balanced for healthy, clean skin!
Dove Soap pH test | Must watch before you purchase | #shorts #phtest #phtesting #shortvideo
Frequently Asked Questions
What is the pH level of soap?
Soap typically has a pH level between 8 and 10, making it slightly alkaline. This pH range helps soap effectively cleanse oils and dirt from the skin without causing irritation.
How does the pH of soap affect its cleaning properties?
The alkaline pH of soap allows it to interact with oils and grease on the skin’s surface, breaking them down for effective cleansing. This pH level helps maintain the skin’s natural balance while removing impurities.
Can using soap with a high pH level be harmful to the skin?
While soap with a higher pH can be effective in cleaning, prolonged use may disrupt the skin’s natural acidic barrier, leading to dryness or irritation. It is recommended to choose a soap with a balanced pH level to support healthy skin.
Why is it important to consider the pH level of soap for skincare?
The pH level of soap plays a crucial role in maintaining the skin’s acid mantle, a protective barrier that defends against bacteria and environmental factors. Using a soap with a pH that aligns with the skin’s natural acidity can help preserve its health and hydration.
Final Thoughts
In conclusion, the pH of soap is typically alkaline, ranging between 8 and 10. This pH level makes soap effective at cleaning by breaking down grease and dirt. The alkaline nature of soap helps it to emulsify oils and remove impurities from surfaces. Understanding what pH is soap can help you choose the right product for your cleaning needs.